Introduction
Given that the overwhelming majority of biopharmaceuticals are proteins, this article will explore some key points on protein structure. This is particularly important because protein function is directly related to protein structure. Specifically we will look at the different types of molecular bonds found in proteins and their relationship to the final three-dimensional configuration of said proteins.
“…protein function is directly related to protein structure”
If we are to appreciate the profound sensitivity of proteins to environmental conditions, we must have at least a superficial understanding of these structure-function issues. This intrinsic sensitivity of proteins to environmental conditions has direct impact on biopharmaceutical storage, transportation and ultimately safety. In the coming weeks all of these fundamental issues will be addressed more directly as they pose some of the greatest challenges to utilization of biopharmaceuticals in our region.
The Nature & Role of Chemical Bonds in Protein Structure
As mentioned in the earlier post, “What is a Biopharmaceutical? (Part II): Biopharmaceutical Characteristics“, proteins are most often very large molecules with highly intricate three-dimensional structures. These complex three-dimensional structures are made possible only through an elaborate network of different types of chemical bonds. The two (2) main classes of chemical bonds that play a role here are ‘covalent’ and ‘non-covalent bonds’.
The Role of Covalent Bonds
Covalent bonds are relatively strong chemical bonds resulting from the sharing of pairs of electrons between multiple atoms. This is likely a good time to remind those of us that were never excited by the prospect of another chemistry class that electrons are negatively charged sub-atomic particles…enough said. The primary structure of proteins is maintained exclusively by covalent bonding. A special type of covalent bond called a ‘Cysteine Bridge’ (Crommelin and Jiskoot, 2008:8) which is named after the amino acid involved, also contributes to the maintenance of the secondary and tertiary structure of proteins. Secondary and tertiary protein structure will also be elaborated upon later in this post.
Share this Post
The Role of Non-covalent Bonds
Non-covalent bonds on the other hand are chemical bonds that do not involve the sharing of electrons. In this case, the structural formations that interact through non-covalent bonding, do so as a result of electrostatic forces. In simple terms, this is a case where opposites do really attract, so negatively charged formations are attracted to positively charged formations in much the same way that magnets of opposite polarity stick to each other. Likewise, structural formations with similar charges repel each other, in the same way that magnets with similar polarity repel each other.
Non-covalent bonds include a variety of bond types. The most important of these in relation to protein structure are, ‘hydrogen bonds’, ‘van der Waals forces’ and ‘hydrophobic interactions’ which are all relatively weak forces. These bond types are integral to the secondary and tertiary structure of proteins. (Crommelin and Jiskoot, 2008:8)
Getting More Specific with Proteins
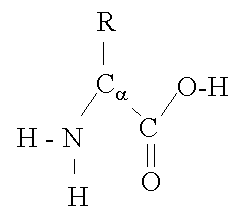
Figure 1: Basic Amino Acid (AA) Structure The above captioned image was originally published on the Wikipedia.com website: http://en.wikipedia.org/wiki/File:A-amino-acid.png
The basic subunits or building blocks of proteins are ‘amino acids’ often abbreviated ‘AA’s’. The AA structural backbone can be standardized for the purposes of explanation as seen in Figure 1. Variation in individual AA structure is then manifested only through differences in the attached side-group at location ‘R’ in Figure 1. The other sections of amino acid structure do not vary from AA to AA.
Protein Size
There is significant variation in size between proteins. Some proteins contain less than 100 AA’s while others may contain tens of thousands of AA’s. This crude size comparison in itself speaks to the huge differences from one protein to the next. Compare biopharmaceuticals to much smaller conventional chemical medicines and the differences in size are even more striking. As an example, proteins are generally in the order of 100 -1000 times larger than conventional chemical medicines. (Kuhlmann and Covic, 2006:5) The table below provides a more practical illustration of this and highlights the dramatic differences in size between these two classes of drugs:
|
Chemical Drug
(Chemical)
|
Molecular Weight (Daltons) | Biological Drug (Protein) | Molecular Weight (Daltons) |
|
metformin
|
166 | Somatropin |
22,125
|
| paclitaxel | 854 | Factor VIII |
264,000
|
(Table adapted from EuropaBio, cited in Kuhlmann and Covic, 2006:4)
Conclusion
Having looked in this article at the underlying forces influencing protein structure, in our next post, ‘Protein Structure – Part 2’, we will define in greater detail the four (4) levels of protein structure and briefly look at how the complexities of this structure result in the highly delicate nature of proteins.
References
1. Crommelin, D.J.A. (2003), ‘Differences between biopharmaceuticals and low-molecular-weight pharmaceuticals’, European Journal of Hospital Pharmacy (1) 2003, pp. 74-75.
2. Crommelin, D.J.A (2008), ‘Some considerations of shifting paradigms in pharmacy’. In: Schellekens, H. & Vulto, A.G. (ed.). Biopharmaceuticals for European Hospital Pharmacists. Belgium: pharma Publishing and Media Europe, pp. 8-10 2008.
3. Kuhlmann, M. and Covic, A. (2006), ‘The Protein science of biosimilars’, Nephrology Dialysis Transplant, (2006) 21 [Suppl 5], pp.v4-v8. DOI: 10.1093/ndt/gfl474



14 Comments on “Biopharmaceutical Protein Structure – Part 1”
I learned a lot from this post, great help for me, thank you!
Hello could I quote some of the insight from this entry if I link back to you?
Sure!!!
Hi Katherine glad you found it useful I’ll do my best to keep the standard up.
Happy to hear it Julio please stick around.
Stewart, I had only read the first entry before but caught up on the rest today. We both know I’m not exactly a science brains but regardless I was riveted. This is really interesting stuff and important knowledge for anyone susceptible to illness – meaning everyone!
Thanks Maria glad you think so. I hope that means you’ll be a regular.
I have a question too. Reading about how complicated the structure of these medicines are in comparison to conventional medicines makes me realise that they must be very expensive to produce. Would drugs like these have a place in a country like Jamaica that’s not first world and with very limited funds? What’s the reality for people a country like that benefiting from these drugs?
Hi Again Maria. Your comment is spot on. These drugs do generally cost more than conventional chemical meds the result being that access to them is even more limited than is the case with conventional. As you yourself pointed out this is a very real concern in the developing world. Frankly this is one of those topics that has a lot more room for development so you can count on seeing some articles related to the cost of these meds and finding appropriate solutions to ther use and access within resource-constrained environments such as the Caribbean. Thanks for the interest and stay tuned, your topic will definitely come back and with many persons chosing to comment I’m sure.
Very interesting ideas! I’ll be back for your new articles!
Like it … very well pointed!
Really great post, I’ll definitelly come back on your website.
I read your site often and I just thought I’d say keep up the amazing work!
Whats up! You some sort of professional? Great message. Can you inform me the right way to subscribe your weblog?