Introduction pH is a measure of Hydrogen ion concentration and can also be described as ‘A measure of how acidic a solution is‘. Low pH (0-3) environments are most acidic while high pH (11-14) environments are least acidic and described as alkaline or basic. As mentioned previously, protein structure is dependent on a combination of chemical bonds and electrostatic (Stationary … Read More
Biopharmaceutical Storage & Handling: Solutes & Solvents
As noted in earlier posts such as ‘Biopharmaceutical Protein Structure – Part 1‘, the elaborate 3-dimensional structure of proteins is maintained by a complex interplay of chemical bonds and electrostatic interactions. These electrostatic interactions primarily involve the charged side-groups of the protein’s constituent amino acids. As seen in Figure 1 below, the ‘R‘ groups which represent clusters of atoms unique to each … Read More
Biopharmaceutical Manufacturing – Part 2
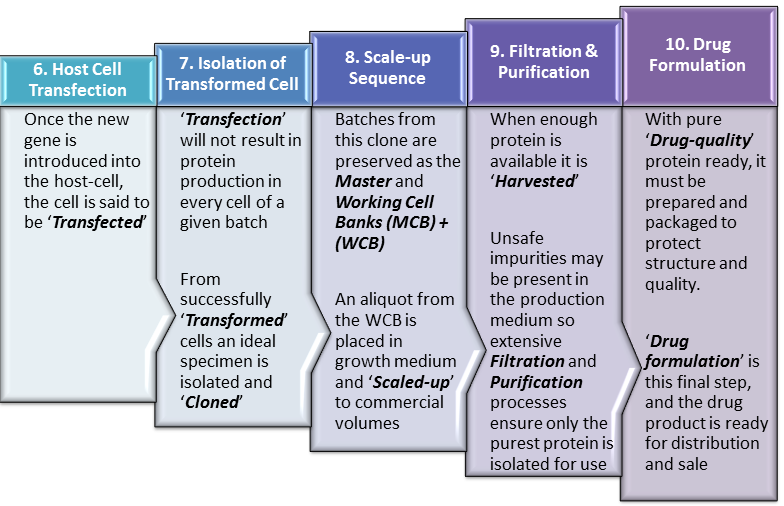
Continuing from our last post, ‘Biopharmaceutical Manufacturing 1‘, this current post, ‘Biopharmaceutical Manufacturing 2‘, elaborates upon the steps in biopharmaceutical manufacture after insertion of the ‘gene of interest‘ into the ‘Expression Vector‘. We therefore begin this post at Step 6 of our Manufacturing Process Flow in which the ‘Vector‘ transports our gene of interest into the host cell. This is presented in even greater detail … Read More
Biopharmaceutical Manufacturing – Part 1
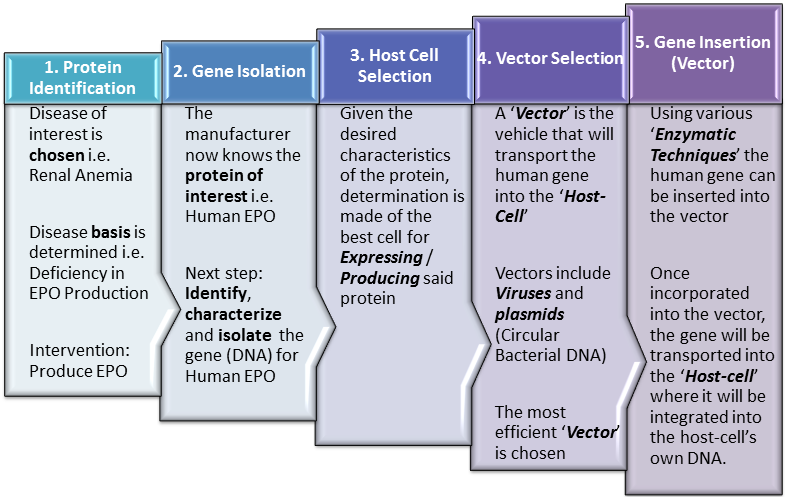
Introduction As noted in earlier posts, because biopharmaceutical manufacturing is dependent upon production within living cells, it is more complex and prone to variability than production of conventional ‘small-molecule‘ / ‘chemical‘ pharmaceuticals. The number of steps involved as well as the complexity of each step, present many opportunities for variability to enter the production process. In fact, minimizing this variability is perhaps the greatest … Read More
What is the ‘Cold Chain’?
The pervasiveness of biopharmaceuticals (biologics) in the 21st century pharmaceutical landscape, means that the presence of ‘heat-labile’ pharmaceuticals even in the ‘corner-store’ pharmacy is almost guaranteed. It is therefore essential that every pharmacist and all dispensary staff have some basic knowledge on how to store, handle and transport Cold Chain Products (CCPs). Cold Chain Products (CCPs) are those requiring storage under refrigerated conditions of 2-8°C. … Read More