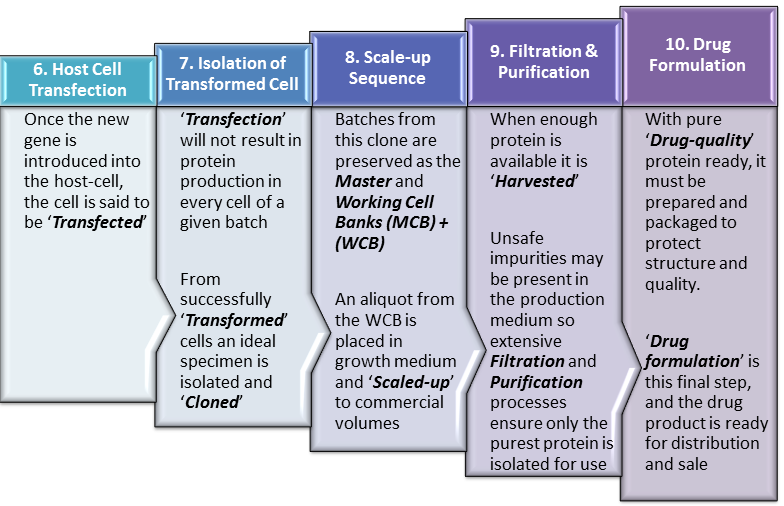
Continuing from our last post, ‘Biopharmaceutical Manufacturing 1‘, this current post, ‘Biopharmaceutical Manufacturing 2‘, elaborates upon the steps in biopharmaceutical manufacture after insertion of the ‘gene of interest‘ into the ‘Expression Vector‘. We therefore begin this post at Step 6 of our Manufacturing Process Flow in which the ‘Vector‘ transports our gene of interest into the host cell. This is presented in even greater detail … Read More
Biopharmaceutical Manufacturing – Part 1
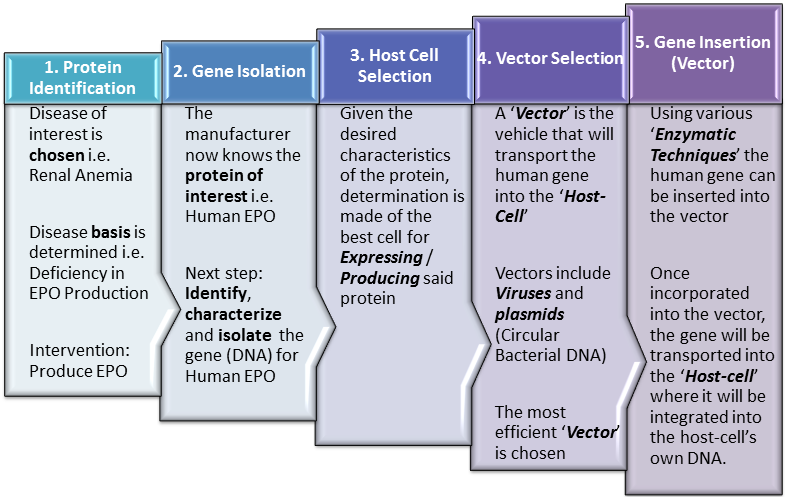
Introduction As noted in earlier posts, because biopharmaceutical manufacturing is dependent upon production within living cells, it is more complex and prone to variability than production of conventional ‘small-molecule‘ / ‘chemical‘ pharmaceuticals. The number of steps involved as well as the complexity of each step, present many opportunities for variability to enter the production process. In fact, minimizing this variability is perhaps the greatest … Read More
Classification of Pharmaceuticals: Biologics vs. Conventional Medicines
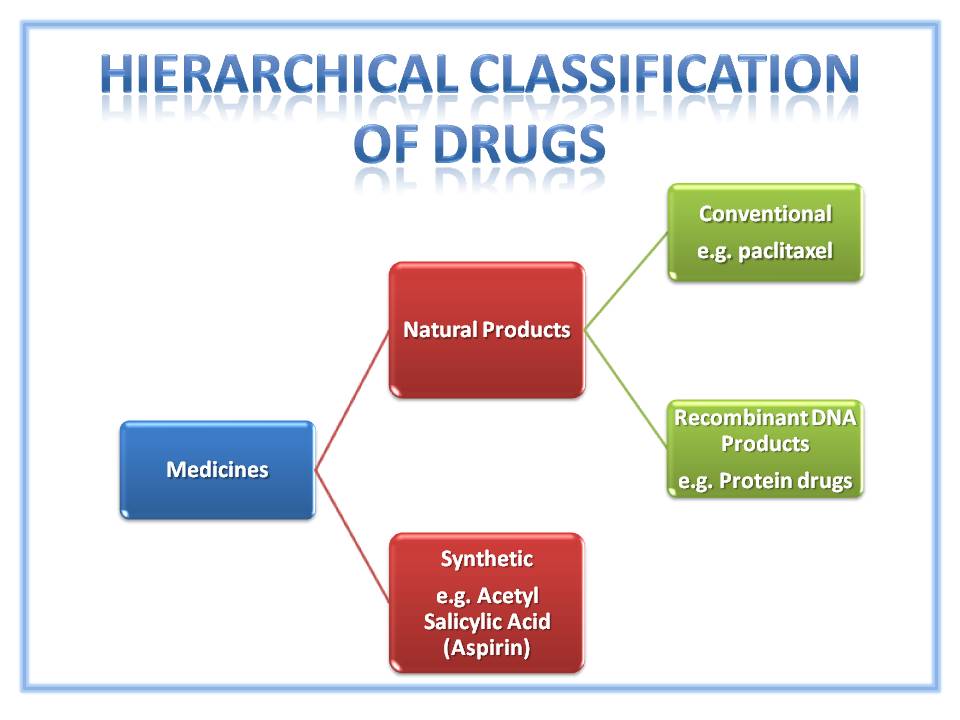
Introduction Medicines can be separated into two broad groups. The first is ‘Synthetic Medicines‘. As the name suggests they are created through supervised chemical synthesis. The associated chemical reactions are relatively simple and often sequential. The final active substances produced are small molecules with relatively simple chemical structures, an example being aspirin (Figure 2). Natural Products By contrast natural products are derived from comparatively complex starting materials sourced from the ‘Natural Environment‘. More specifically, they are derived from … Read More