Introduction
The phrase ‘The Product is the Process‘, encapsulates the unique relationship between the production process and the characteristics of the resultant biopharmaceutical product. This inextricable relationship between the process and the product is not seen with the production of conventional chemically-derived drugs. With conventional drugs, it is not uncommon for the sequence of production steps to be altered with little effect on the final product. To the contrary, the average biopharmaceutical manufacturer, is unlikely to even consider changing the sequence of steps in the production process, recognizing that the effect on the final product is likely to be unpredictable and possibly profound.
The phrase ‘The Product is the Process‘ as used by industry insiders, therefore seeks to capture this nuance of biopharmaceutical production, wherein even minute changes to the production process will be manifested in the final biopharmaceutical product. Furthermore, because the structural complexity of biopharmaceuticals makes their full characterization so elusive, the simplest means of ensuring consistency from ‘batch-to-batch’, is to instead, fully characterize the production process from beginning to end, then uncompromisingly execute those steps without variation. In biopharmaceutical manufacturing therefore, ‘the process becomes as much a product, to be patented and protected as the resulting drugs themselves‘.
In biopharmaceutical manufacturing therefore, the process becomes as much a product, to be patented and protected as the resulting drugs themselves.
In Biopharmaceutical Manufacturing 1 & Biopharmaceutical Manufacturing 2, we discussed in some detail, the highly complex and variability-prone nature of biopharmaceutical production. This reality obtains primarily because biopharmaceuticals by definition, are produced within living cells and being dynamic systems, cells adjust continuously to their environment. Given the previously stated points, this implies that if conditions change during production, so too will process outputs inclusive of the biopharmaceutical product itself, so once more ‘The Product is the Process‘. It is therefore a significant challenge for any manufacturer to ensure ‘batch-to-batch‘ consistency even with a well-defined process and detailed records. Actually achieving this, requires strict oversight, stringent process controls and prolific record-keeping at every step.
Share this Post
The Biopharmaceutical Production Process – Unique to Each Product
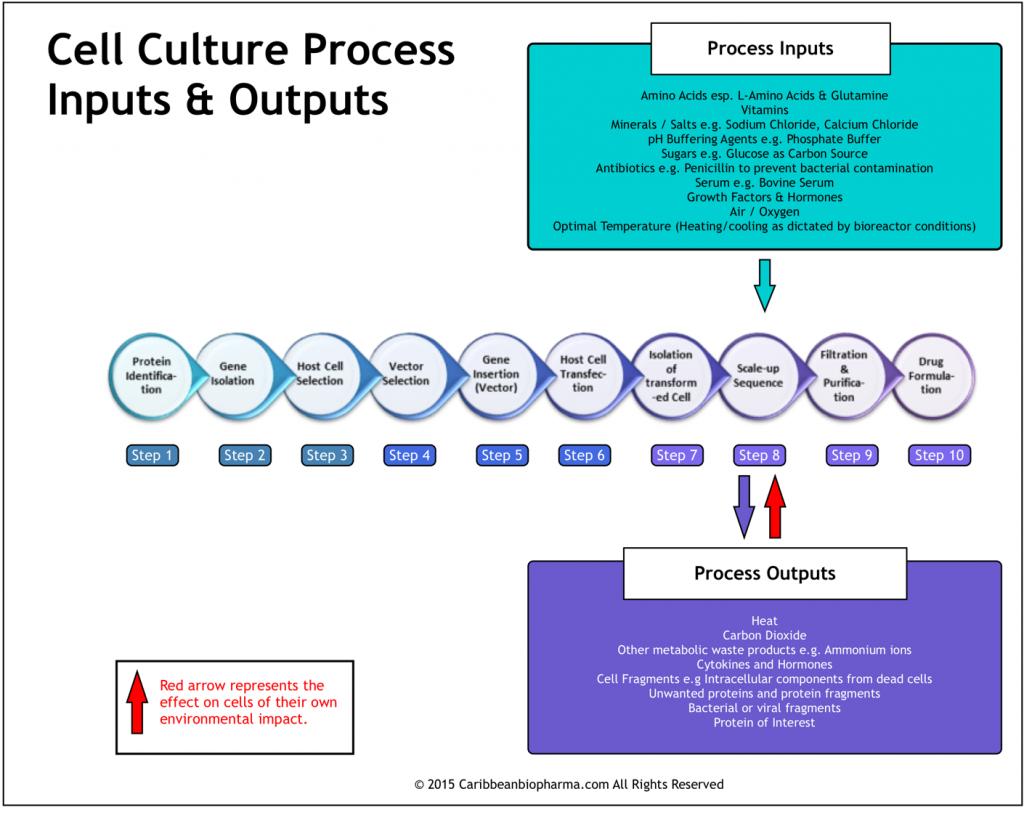
As stated in other posts, production of a given biopharmaceutical, incorporates steps that are themselves, unique occurrences not found with other biopharmaceuticals. As an example of this uniqueness within the production process, we can look at the ‘Host Cell Transfection‘ phase of biopharmaceutical development (See Biopharmaceutical Manufacturing 2 for more details). Host Cell Transfection is a unique exercise for each biopharmaceutical developed and the outcome is dependent upon variables such as the ‘The Transfection Vector chosen‘, ‘The Type and Strain of Host Cell Selected‘, ‘The Gene Transferred‘, among other factors (See steps 2-6 in Figure 2 below). The ‘distinct’ set of elements combined in this phase of production, yields a unique population of genetically-engineered cells, cloned from a single ideal specimen. This population of cells constitutes the Master Cell Bank or MCB.
The cells of the Master Cell Bank will be the source of all cells used to manufacture the specific biopharmaceutical over the entire lifetime of that product and in fact, they have the single greatest impact of any process component on the nature of the final product. This is because the characteristics of the MCB not only dictate the types of inputs required during the cell culture process (during which the protein of interest is produced), but also strongly influence other fundamental variables as well. These other variables include; the quantity of the specified inputs mentioned above, the types of vessels to be used, the ideal form of agitation employed in the bioreactor, the filtration and purification processes used, among many others.
The Profound Impact of Changes to the Cell Culture Environment
Ultimately, the characteristics of the final protein are significantly affected by the cells’ response to their environment during the ‘Cell Culture Phase‘ (Scale-up Sequence in Figure 2). Importantly, the environment in turn, is affected not only by process inputs but also by the cells’ own activity, in the form of process outputs. Highlighting this, Figure 2 shows that in addition to the ‘protein of interest‘, the cells also produce waste substances such as carbon dioxide and ammonium ions while also generating heat as a by-product of metabolic activity. These outputs themselves, impact the environment in which the cells exist and this in turn comes full circle, affecting the cells’ behaviour. This interaction elegantly highlights just how complex the biopharmaceutical production process can be.
An examination of the number of steps involved in the process, each with its own set of inputs and requirements for optimization, reveals even greater depths of complexity. With the plethora of factors to be controlled, the possibilities for process variations are rife, more so because a single change, theoretically can have a profound impact, potentially leading to unacceptable product changes.
Production Monitoring and its Impact on the Process
As alluded to above, the nature of the MCB will influence not only the final product but also the impurities and waste products emerging during production. Ultimately this means that the MCB also affects the type, sequence and frequency of analytic assessments needed to monitor production. Furthermore, the approach taken in monitoring, is likely to be updated and perfected progressively over time with ‘trial-and-error’ also playing a part. In the end, the effectiveness of the analytic approach taken, will also shape the ‘Filtration and Purification‘ scheme employed and will ultimately impact the consistency, purity and overall quality of the final product. Given its importance, the specifics of the analytical approach employed, will constitute part of an organization’s intellectual property. Significantly, if the analytical approach is changed, this can represent a further point through which variability can be introduced into the final product, again highlighting the critical impact of changes anywhere along the production chain.
Conclusion
While a multiplicity of factors exist which affect biopharmaceutical production, the most significant influence on the process is easily the Master Cell Bank; the source of cells actually responsible for producing the desired protein. In this post, we explored the impact of the Master Cell Bank on biopharmaceutical production, how environmental changes impact the growth of the cell culture and ultimately how these changes affect the character of the final product. We also explored the industry catch phrase, ‘The Product is the Process‘ to illustrate the high level of sensitivity associated with the biopharmaceutical process.
Emphasizing the pivotal role of the MCB in biopharmaceutical production, our next post will look at the economic value of the MCB and what losing it can mean for a manufacturer, as well as typical steps put in place to protect this extraordinarily valuable resource.