Introduction
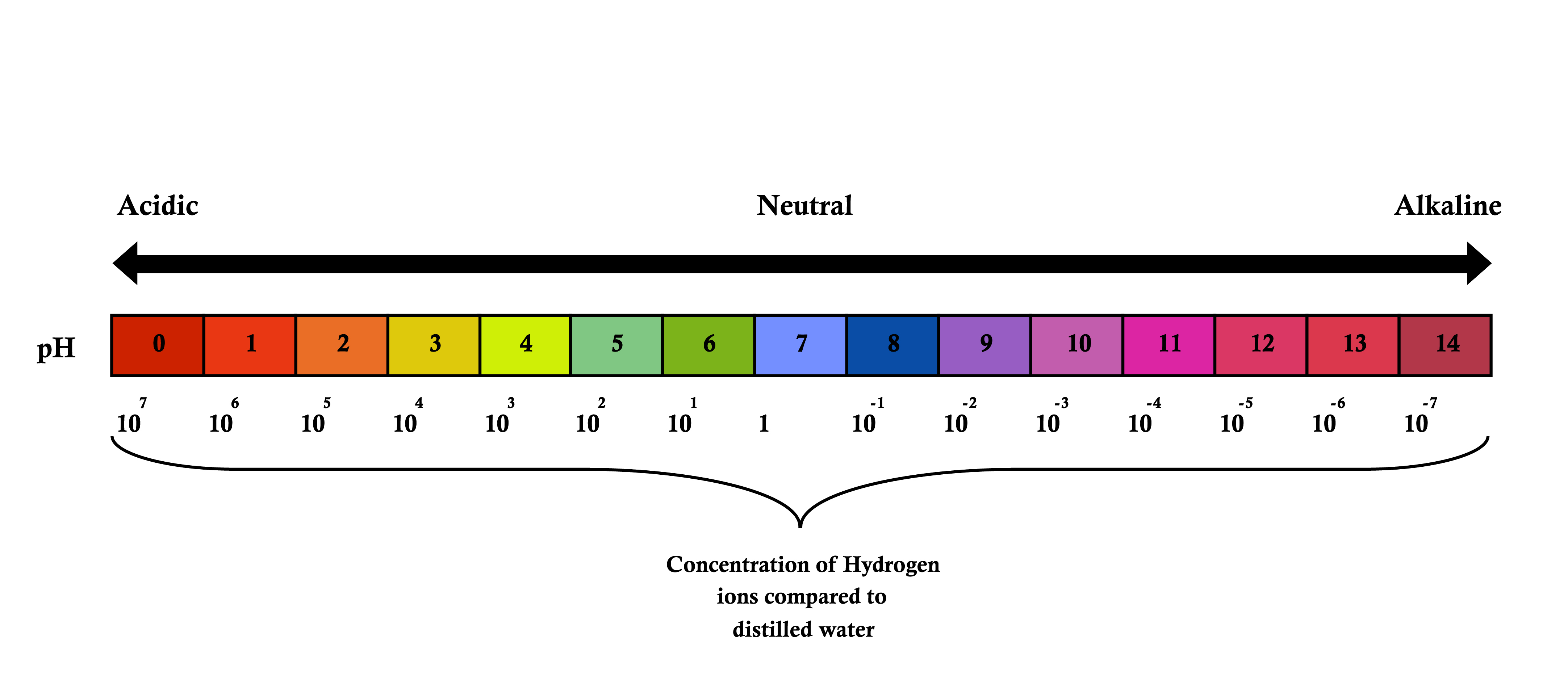
pH is a measure of Hydrogen ion concentration and can also be described as ‘A measure of how acidic a solution is‘. Low pH (0-3) environments are most acidic while high pH (11-14) environments are least acidic and described as alkaline or basic.
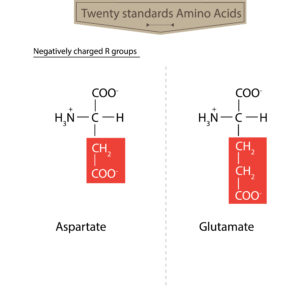
As mentioned previously, protein structure is dependent on a combination of chemical bonds and electrostatic (Stationary Electrical) forces (See Biopharmaceutical Protein Structure – Part 1). The specific electrostatic pattern within a protein, is strongly influenced by the nature, location and interactions between the charged side-groups found on the protein’s constituent amino acids. Significantly, the electrostatic charge of these side-groups can be altered by environmental factors including pH. A direct consequence of this, is that protein structure and behavior are profoundly impacted by pH as well as other factors.
Share this Post
Native Protein Structure (pH-Neutral Conditions)
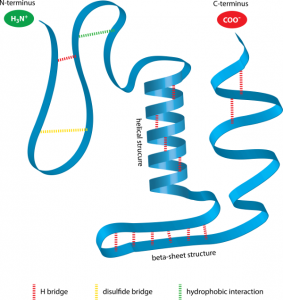
The environment within the human body is considered neutral with a pH of 7.4. Under these pH-balanced conditions, proteins most often exist in their optimal configuration. In this form, specific negatively- and positively-charged side-groups along the protein chain, attract each other in an attempt to achieve electrical neutrality. In this sense they behave much like magnets of opposite polarity. When this occurs, the long protein chain bends and folds where these oppositely charged side-groups come together resulting in the characteristic shape of that protein (See Figure 2).
It is useful at this stage to point out that not only interactions of oppositely-charged side-groups impact upon protein configuration. To the contrary, similarly-charged side-groups also interact along the length of the protein chain. When similarly-charged side-groups interact they instead repel each other like magnets with the same polarity and in so doing have their own impact on protein structure.
The sum total of all these attractions and repulsions along the length of the protein chain, yields the final functional shape of the protein. Achieving optimal protein configuration therefore, is a highly complex, multi-variable process.
How Does a Change in pH Affect Protein Configuration?
To illustrate how pH alterations change protein configuration, we can examine the same protein described above, this time under acidic conditions. By definition, positively-charged Hydrogen ions are more abundant in acidic environments. This abundance means for example, that negatively-charged side-groups on the protein chain are now more likely to interact with these hydrogen ions and not with the positively-charged side-groups it ordinarily would.
The altered interaction between the usual side-groups means the protein will not twist and fold at the same points, or in the same way that it did in the pH-neutral environment. This will result in the protein assuming a different final configuration. Exposure to alkaline conditions will alter the side-groups in yet other ways than either neutral or acidic conditions, yielding another set of structural variants.
Acidity also affects proteins in another significant way. Extremes of acidity can irreversibly damage proteins, permanently reducing their activity. This is because high acidity causes hydrolysis or cleavage of the chemical bonds joining amino acid residues together in the protein chain. When these bonds are broken, the protein is effectively cut into small non-functional fragments. In the best of cases these fragments may simply be ineffective in treating the condition for which the protein drug was intended. In other cases, these fragments may actually be harmful to the patient, causing immunogenic or other deleterious effects.
Conclusion
It is universally accepted that protein function is inextricably linked to protein structure. So anything that alters protein shape, by default, will alter protein function and activity. The take home message therefore, is that if amino acid side-groups are exposed to conditions other than those of their native environments, the protein as a whole will be configured differently and as a result, behave differently. In some instances these change will be reversible but in others less so.
In the end, pH (acidity) like so many other factors, critically influences protein structure and by extension, protein function and safety. If patient safety is to be preserved therefore, precautions must be taken to ensure that protein drugs are not exposed to conditions outside of those recommended, pH being only one of many such considerations.